Abstract
Fin whales (Balaenoptera physalus quoyi) of the Southern Hemisphere were brought to near extinction by twentieth century industrial whaling. For decades, they had all but disappeared from previously highly frequented feeding grounds in Antarctic waters. Our dedicated surveys now confirm their return to ancestral feeding grounds, gathering at the Antarctic Peninsula in large aggregations to feed. We report on the results of an abundance survey and present the first scientific documentation of large fin whale feeding aggregations at Elephant Island, Antarctica, including the first ever video documentation. We interpret high densities, re-establishment of historical behaviours and the return to ancestral feeding grounds as signs for a recovering population. Recovery of a large whale population has the potential to augment primary productivity at their feeding grounds through the effects of nutrient recycling, known as 'the whale pump'. The recovery of fin whales in that area could thus restore ecosystem functions crucial for atmospheric carbon regulation in the world's most important ocean region for the uptake of anthropogenic CO2.
Similar content being viewed by others
Introduction
Fin whales (Balaenoptera physalus quoyi) of the Southern Hemisphere were brought to near extinction by twentieth century industrial whaling1. More than 700,000 individuals were killed2 between 1904, when intensive commercial whaling began in the Southern Ocean, and 1976, when the catch quota of fin whales was set to zero, 10 years before the moratorium on whaling3,4. It has been estimated that by then the population had been reduced to 1–2% of its pre-exploitation size of around 325,000 animals in the early twentieth century2,5,6. Major whaling for fin whales took place at their feeding grounds at the northern tip of the Antarctic Peninsula7. After the end of commercial whaling, cetacean surveys conducted under the auspices of the International Whaling Commission (IWC) between 1978 and 2004 (IDCR/SOWER surveys), reported very few fin whales in that region8. They had seemingly vanished from those historical feeding grounds. About 40 years later, our surveys now confirm a return of the whales to their ancestral feeding grounds in high numbers, forming large feeding aggregations.
In the post-whaling era, the International Whaling Commission’s (IWC) International Decade of Cetacean Research (IDCR) and Southern Ocean Whale Ecosystem Research (SOWER) cruise programmes, carried out in three circumpolar sets of surveys between 1978 and 2004, provided the only comprehensive information on fin whale abundance in the Southern Hemisphere. Based on IDCR/SOWER data from surveys between 1991 and 1998, circumpolar fin whale abundance south of 60°S was estimated at 5445 (95% CI 2000–14,500)8. However, since the surveys did not cover the full latitudinal distribution of fin whales and an unknown proportion of the population will have ranged north of 60°S even in summer, this estimate almost certainly represents an underestimate. For the Scotia Arc and Antarctic Peninsula region, fin whale abundance was last estimated at 4672 (CV 42.37) based on data from the CCAMLR/SOWER survey conducted in 20009. Globally, fin whales are listed as 'vulnerable' on the IUCN Red List of Threatened Species10. Their status was changed from 'endangered' to 'vulnerable' in 2018 based on projections of the global mature population size. It was however noted that global population estimates were associated with much uncertainty due to lack of data especially from mid-latitudes in the Southern Hemisphere10.
Since the 2000s, observations of fin whales from the Antarctic Peninsula region have been increasing. First, surveys using platforms of opportunity traveling between South America and the Antarctic Peninsula in 2001 and 2002 indicated considerable densities of fin whales in the offshore waters running parallel to the Antarctic Peninsula. Shortly after, Santora et al.11 suggested hotspots of fin whale occurrence in the Southern Drake Passage and around Elephant Island12 based on high encounter rates recorded during krill surveys between 2003 and 2011. In 2012, an opportunistic observation of an aggregation of more than 100 animals was reported13,14. A year later, an aerial cetacean survey around the South Shetland Islands provided an abundance estimate of 4898 (95% CI 2221–7575) fin whales (survey area: ~ 42,000 km2; density = 0.117; 95% CI 0.053–0.181 individuals/km2), with observations of fin whales feeding in groups of up to 70 animals15. Lastly, in 2016, a shipboard cetacean survey reported high fin whale densities around Elephant Island (0.0268 ± 0.0183 individuals/km2) and the South Orkney Islands (0.0588 ± 0.0381 individuals/km2)16. These consistently high numbers were our motivation for a dedicated assessment. We therefore conducted two surveys (a shipboard survey and a vessel-supported helicopter survey) around the northern tip of the Antarctic Peninsula to estimate fin whale abundance and to investigate the new phenomenon of fin whale feeding aggregations.
In this paper, we report on the results of an abundance survey and present the first documentation of fin whale feeding aggregations. We discuss the ecological implications of the recovery of a large baleen whale species and its return to ancestral feeding grounds against the background of ecosystem services provided by whales.
Methods and fieldwork
Data collection
Data were collected during two expeditions to the Antarctic Peninsula in 2018 and 2019.
During the multidisciplinary research expedition PS112 (18 March–5 May 201817) of the German research ice breaker Polarstern18, we conducted a vessel-supported helicopter survey [using the on-board helicopter (BO-105)] to estimate abundance of fin whales along the northern tip of the Antarctic Peninsula. Data collection followed line transect distance sampling methodology19 and an adaptive ad-hoc survey design15,20. Owing to logistics of a multidisciplinary research cruise, where the ship simultaneously caters to the needs of several research projects on board21, it was impossible to follow a pre-designed fixed survey scheme. Instead, aerial transects were placed around the current position of the ship, aiming at an adequate overall coverage of the survey area and applying basic principles of good survey design following Buckland et al.19 (i.e., arbitrary orientation and placement of transects with respect to whale distribution). Based on this ad-hoc method (described in more detail in Herr et al.15), our survey was planned in anticipation of model-based abundance estimation22,23,24 rather than conventional design-based analysis24,25. Flight altitude was 600 ft at a survey speed of 80–90 knots. Two experienced observers (the same throughout the whole survey) seated in the front and back left seats of the helicopter collected sighting data. The front observer covered the area directly below the helicopter, i.e. the transect line and up to 80 m to the left, through the bottom front window. The back observer covered the remaining area to the left of the transect line up to the horizon. Together, both observers provided full coverage of the left side of the transect line and were treated as one observer during analysis. To avoid potential duplication of sightings, continuous communication was maintained via intercom. Coverage of only one side of the transect was accounted for during analysis (i.e. detection function modelling, see below). All data were entered directly into a computer running dedicated data collection software (VORaudio, designed by Lex Hiby and Phil Lovell), continuously storing GPS data obtained via a GPS device (Garmin 72H). Sighting conditions were judged by the observers and information entered with every change therein. The following two measurements were used: sea state in the Beaufort scale and 'subjective sighting conditions', i.e. the observers' evaluation of the chances to detect an available large whale (a compound variable taking the levels 'good', 'moderate', and 'poor'). For each sighting, the species, declination angle from observer to animal, and group size were noted. Declination angles were measured using inclinometers and were used to calculate distances of sightings to the transect line.
Survey flights were accompanied by a camera operator to film fin whale encounters, and particularly, feeding events. Additional sightings of fin whale aggregations were documented opportunistically during ship transit, logging position and group size.
In 2019, the Pelagic Australis expedition revisited the survey area of the PS112 expedition. This expedition was dedicated to media purposes and the documentation of fin whale feeding aggregations, therefore a line transect survey was not conducted. Fin whale aggregations were explicitly searched for and encounters were recorded and filmed.
Video documentation
Video imagery in 2018 was collected from the helicopter, from the RV Polarstern deck and using drones. In 2019, videos were made from drones and an inflatable powerboat, both deployed from the Pelagic Australis. A stabilised camera system was attached to the helicopter, using a RED Helium 8 K camera with Canon CN20 (50–1000 mm) lens inside a GSS gyro-stabilised system to film during helicopter flights. The same system was used to film from the deck of the ship. If feeding aggregations were encountered during ship transit, drones (DJI Phantom 4 and Inspire II equipped with a Zenmuse X5S camera) were launched to collect aerial imagery.
Analyses
We used all sighting records of fin whales (not including any aggregations) collected during helicopter survey effort in a distance sampling analysis for abundance estimation19,23,25. First, we modelled a detection function to account for the effects of distance and other covariates on the detection probability of whale groups. We used the R software package ‘Distance’26 for a multiple covariate distance sampling (MCDS) analysis27, assuming that the probability of detection on the track line was 1 (i.e., 100%). Mean group size was estimated via the regression method19 to account for effects of group size bias on detectability. In theory, at large distances large group sizes are more likely to be detected than small groups or single animals. Therefore, this bias can be accounted for by estimating a correction based on the regression of group sizes with distance. Sighting data were manually right-truncated at 1750 m after visual inspection of the distribution of sightings to exclude outliers at large distances. We used sea state and subjective sighting conditions (i.e., 'good', moderate' and 'poor') as potential covariates in a half-normal and hazard-rate detection function model, including a cosine adjustment series of order 2 for the half-normal and no adjustment for the hazard rate models. Model selection was based on Akaike’s information criterion (AIC,28) and the model’s capability to accurately capture the number of sightings near the transect line p0 and Goodness of fit as expressed by the Cramér van Mises test statistic.
For the density surface model, we aggregated effort and the number of fin whale groups and individuals to segments of 5 km length along the transect lines (sometimes resulting in segments < 5 km at the end of transects or effort and discarding all segments with a total length < 1 km) and calculated the effectively covered area within each segment as:
With \({A}_{seg}\) the effectively covered area [km2] along segment seg, \(esw\) the effective half strip width [km] based on the detection function model and \({L}_{seg}\) the effort [km] along segment seg.
We then used the ‘mgcv’ package29,30 to fit an additive model of the observed fin whale groups per segment, off set by the log of the effectively searched area per segment, to a smoothed interaction of x and y (projected longitude and latitude values of segment midpoints, respectively) and combinations of x and y with water depth (IBCSO v231) and derived properties TPI (topographic position index)32, TRI (terrain ruggedness index)32, slope and aspect. TPI, TRI, slope and aspect were calculated from the depth raster using the ‘raster’ package33. Segment covariates were extracted along each segment and averaged for the whole segment. Additional covariates tested in the models were the calculated distance from the segment midpoints to the shelf break (as defined in Herr et al.20) and to the nearest coastline. Since our main interest was a simple and robust snapshot of abundance and distribution rather than an ecological model describing drivers of distribution, we included variables in addition to x and y only to test if they improved the simplest model containing only x and y34. Since this was not the case, we did not include any interactions between the covariates and no testing for correlation between covariates was needed.
We used a thin plate smoother for all terms and an (auto starting) Tweedie error distribution for the model residuals30,35. Final model selection was based on AIC28, their generalised cross validation score (GCV36) and deviance explained. In case of similar model performance, we opted for the simpler model, i.e. the model with fewer covariates.
We covered the surveyed area (i.e. the area within the survey boundary, Fig. 1) with a grid of 2.5 × 2.5 km cells, attributed with the same covariates as used in the density surface model, and predicted the number of fin whale groups per grid cell across this area. We assessed the CVs associated with the predictions in every cell and discarded cells with CVs ≥ 100, limiting the spatial extent of the prediction area to areas supported by sufficient data coverage and avoiding extrapolation beyond reasonable boundaries. The remaining area served as the prediction area for which we estimated fin whale abundance based on the model. To translate group density results to individual abundance we multiplied the number of groups by the group size of fin whales estimated from the regression of original sighting records across all observed distances. The total abundance was based on the sum of abundance of all cells within the area. We used the standard error as reported by the model for the calculation of confidence intervals across the whole study area. All analyses were done in R version 3.6.1 (R Core Team 2019). Imagery was used to document feeding aggregations and behaviour, and to aid group size estimation of large feeding aggregations. We defined feeding behaviour as a display of lunges, repetitive and consecutive diving behaviours and expanded buccal cavities at surfacing. We use the term aggregation to describe groups of 15 or more individual whales estimated to be within five body lengths of their nearest neighbour.
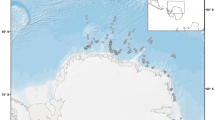
Survey effort and fin whale sightings. Representation of transect lines covered by the aerial survey during RV Polarstern expedition PS112. Fin whale sightings recorded on-effort during the aerial survey are indicated as yellow dots. Positions of fin whale aggregations (stars) comprise sightings collected during both expeditions. The map was composed using ESRI ArcGIS 10.6 (https://www.esri.com/en-us/arcgis/products/arcgis-desktop/resources).
Results
Fin whale records and group sizes
During the aerial survey of expedition PS112, we completed 22 survey flights between 23rd March and 24th April 2018, covering 3251 km of track lines during 26.3 h of search effort. We recorded 100 groups of fin whales on effort (Fig. 1), with group sizes ranging from 1–4 individuals and a mean group size of 1.28 ± 0.04 animals. No aggregations were encountered during aerial survey effort and thus no records of aggregations were included in the distance analysis. A small aggregation of 15 animals was encountered off-effort (i.e., during helicopter transit to a transect), with fin whales feeding together with Antarctic fur seals (Arctocephalus gazella) and chinstrap penguins (Pygoscelis antarcticus) (Table 1). During ship transit, two aggregations of approximately 50 and 70 animals respectively were encountered (Fig. 2, Table 1). The first one was encountered in a situation interpreted as 'post-feeding' (Online Supplementary Material (OSM) Video 1) with a high density of animals in an active behavioural state, but not feeding. The second aggregation was encountered with intense feeding activity ongoing (Fig. 3, OSM Video 2). Drone footage provided aerial close-up imagery of feeding behaviour (Fig. 4, OSM Video 3). During the Pelagic Australis expedition in 2019, five fin whale aggregations were recorded at Elephant Island (Table 1, Figs. 1, 2), with the largest two counting approximately 150 animals (Fig. 5, OSM Video 4).
Close up view of survey effort and sightings around Elephant Island. All aggregations during both expeditions were recorded at the northern coast of Elephant Island. The map was composed using ESRI ArcGIS 10.6 (https://www.esri.com/en-us/arcgis/products/arcgis-desktop/resources).
Fin whale feeding aggregation. Aerial view on a section of the active feeding aggregation of ~ 70 fin whales encountered during ship transit on RV Polarstern expedition PS112 in 2018, filmed by drone. ©BBC (OSM video 2).
Close-up sections of the active feeding aggregation of ~ 70 fin whales. This aggregation was encountered during ship transit on RV Polarstern expedition PS112 in 2018 and filmed by drone. Fin whales are side-lunge feeding together at the surface. In the bottom panel a single humpback whale feeding with the fin whales is visible. ©BBC (OSM video 3).
Fin whale feeding aggregation at a distance. The horizon is covered by blows of a feeding aggregation numbering approximately 150 fin whales. ©BBC (OSM video 4).
Density and abundance estimation
Sighting data were truncated at a distance of 1750 m. All models using the hazard-rate key were deemed not adequate for the present data, because visual inspection of the histograms (with the overlaid detection probability curve from the hazard rate models) indicated a poor fit at distances near zero, and were therefore not considered as final models. The remaining half-normal models all achieved very similar p0-values (i.e., similar results for detection probability). From these, detection function model c1 using the half normal key with cosine adjustment and no additional covariates was chosen as the best model based on AIC values, Cramér von Mises p values and the high accuracy for p0 (Table 2, Fig. 6). Interactions of covariates were not tested due to insufficient sample size at factor combinations (100 sightings overall). The inclusion of covariates did not improve the detection functions. The effective half strip width was estimated at 576 m.
Based on the assessment of AIC, GCV and deviance explained, we chose model g8 (including a spatial smoother across x and y and the distance to the shelf break as covariates; Table 3) as the best additive model. Model g8 achieved the lowest AIC and GCV values of all models, but was not the model with the highest deviance explained (i.e., not the model with the lowest deviance from the saturated model). While the model with the lowest deviance will certainly represent the sample data better than any other model, it may not generalise well (due to overfitting) and therefore does not guarantee small deviance on independent data. Since differences in deviance between the models were marginal, we chose the model with best AIC and GCV performance (model g8) for our prediction. Using model g8 we predicted fin whale densities over the full extent of the survey boundary (for comparison, prediction performance by all models is shown in OSM, Figure S1). The restriction to predictions associated with a CV < 100 resulted in a prediction area of 92,819 km2, discarding the outer margins of the survey boundary. For this area, the density surface model g8 estimated fin whale abundance at 7909 (95% CI 1047–15,743) animals, corresponding to an average density of 0.085 (95% CI 0.0113–0.1696) animals/km2 (Table 4). The predicted distribution of fin whales showed three centres of concentration along the shelf break west of the Antarctic Peninsula. Particularly high densities were predicted around Elephant Island (Fig. 7). In this hotspot area of 17,038 km2, abundance was predicted at 3618 (888–6525) animals, corresponding to an average density of 0.2123 (0.0521–0.383) animals/km2 (Table 4). Abundance estimates represent minimum estimates since no correction for availability or perception bias could be applied.
Fin whale distribution (left) and associated CVs (right) based on aerial survey data from RV Polarstern expedition PS112. Fin whale distribution as predicted by model g8 including a smooth of x and y and the distance to the shelf break as covariates. The spatial extent of the prediction was confined to only contain predictions with CVs < 100. The maps were composed using ESRI ArcGIS 10.6 (https://www.esri.com/en-us/arcgis/products/arcgis-desktop/resources).
Behavioural observations
The behaviour in aggregations was dominated by feeding, with animals lunging with mouths agape, tight turning and repeated strong vertical diving, resembling a 'feeding frenzy'37,38,39. Surfacing animals displayed expanded buccal cavities (Fig. 4, OSM videos 2 and 3). Although no prey sampling could be carried out within the tightly packed feeding aggregations, krill could be observed at the surface, and echosounder data recorded at the time of the feeding aggregation during transit of PS112 indicated dense Euphausia superba presence in the area (personal observation B. Meyer)17. Presence of other krill predator species (Antarctic fur seals and several species of birds mainly of the family Procellariidae) clearly pointed to availability of prey in the upper water column. On one occasion during PS112 a single humpback whale (Megaptera novaeangliae) was associated with a large feeding aggregation of fin whales (Fig. 4, OSM video 3), during the Pelagic Australis expedition, two humpback whales were observed in a fin whale feeding aggregation.
Discussion
Feeding aggregations
The feeding aggregations documented in our study are among the largest ever reported for baleen whales in scientific literature. Similar in size are only the so called 'super-groups' of humpback whales at the South African and East Australian coasts40,41. Large groups (> 15 individuals) or group feeding events have not been described for fin whales anywhere else in the world. Published observations of fin whale feeding events comprised maximum group sizes of 13 animals42,43. Anecdotal reports from the nineteenth century suggest that, prior to their exploitation, fin whales used to aggregate at their feeding grounds in a similar way44,45. But for the post-whaling period our observed group sizes and the aggregative behaviour are novel. The footage presented in this study is the first documentation of large feeding aggregations of fin whales. It was featured in the 2019 BBC nature documentary ‘Seven Worlds, One Planet’, narrated by Sir David Attenborough, who notes the event as 'the largest congregation of great whales ever filmed'. It has been suggested that the recovery to pre-exploitation numbers allows the re-emergence of behaviours, that, due to extremely low population numbers, had no longer been performed or observed41,46.
Lunge feeding, the dominant behaviour observed in feeding aggregations, is of particularly high energetic cost47,48,49. It is usually performed in areas of high prey density in order to make feeding efficient47. Baleen whales are thought to respond to prey distribution according to both aggregative and feeding thresholds. Aggregative behaviour then is responsive to local prey supply, and feeding occurs above a prey density threshold set by the energetic costs for lunge feeding50. The observations of large aggregations of actively feeding fin whales suggest both criteria to be met around Elephant Island.
Fin whale density was generally high throughout the surveyed area, but unless observed in aggregations, group sizes of fin whales were small (i.e., 1–4 individuals). The observed large feeding events were likely "sparked" by krill occurrences above a certain threshold density, triggering feeding and hence, concentrating fin whales from the wider area in one spot. The mechanism of attraction to the same prey cloud can currently only be speculated about. Maybe, the onset of feeding of a few animals attracts other animals in proximity, a phenomenon called 'local enhancement', often observed in birds, leading to a spontaneous occurrence of a feeding frenzy51. The cues to which fin whales respond to, i.e., visual, acoustic or other factors related to prey concentration however, are not yet known.
Density and distribution
The density of fin whales in the survey area (0.0852 individuals/km2; 95% CI 0.0113–0.1696) was high for a large marine animal52 and particularly high compared to fin whale densities in other areas of the world, that are well known for fin whale occurrence (e.g. Southern California53, West Greenland54, Mediterranean Sea55,56). Within the survey area, fin whales were not evenly distributed, but concentrated in three areas along the shelf break west of the Antarctic Peninsula and particularly in a hotspot around Elephant Island, where an average density of 0.2123 (0.0521–0.383) individuals/km2 was predicted (Fig. 7). Similarly high densities have been estimated during a previous aerial survey around the South Shetland Islands in 2013 (0.117; 95% CI 0.053–0.181 individuals/km2)15, spatially matching one of the other hotspot areas. Although no records of feeding aggregations or large groups were included in the abundance survey data, the identified hotspots represent the areas where feeding aggregations have been observed in this study and previously13,15. These consistencies suggest that high fin whale densities and feeding aggregations are a recurring event at these hotspots. At the same time, the fact that no aggregations were observed during helicopter survey effort supports that the aggregations are spontaneous events lasting for short periods of time only, with animals engaging in a 'feeding frenzy' and then dispersing again to the nearer surroundings. During a dedicated aerial survey, the transects are covered at a survey speed of 80–90 knots, providing very little time for observation of any point along the transect and as such representing a snapshot of animal distribution. Chances to record rare events are limited and, from a methodological point of view, even undesired19. Our aerial survey captured the generally high densities of fin whales in the area without violating basic principles of distance sampling by including observations of extremely large groups. We suggest that the high animal density in the area forms the basis for spontaneous formations of feeding aggregations. These were detected from the ships in our study, which had considerably more observation time and actively searched for aggregations.
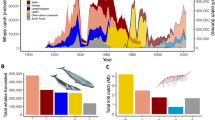
The hotspots identified in our study serve as feeding grounds for fin whales today. Catch records from the industrial whaling period identify the area around the tip of the Antarctic Peninsula as a major whaling ground, where large numbers of fin whales were caught at the beginning of the twentieth century (Fig. 8)7. In that region, the whalers targeted particular areas where they knew fin whales gathered for feeding, and beyond which it was considered needless to look for more once the whales were depleted45. Therefore, the catch records are a good reference for the location of historical feeding grounds. Our results support that fin whales have now returned to at least some of their ancestral feeding grounds.
Culturally inherited site fidelity to feeding and wintering grounds, transmitted through maternally directed learning and fidelity to important habitats57,58,59, is evident in many whale species57,60,61,62,63. The cultural knowledge of habitat as feeding grounds may be lost as a result of extreme population depletion57 and rediscovery is generally very slow, if it happens at all59. Blue whales (Balaenoptera musculus) around South Georgia had been assumed to have been depleted beyond a point of recovery57 and had disappeared completely from their former feeding grounds. Five decades later, increasing numbers of sightings and acoustic records have indicated a return of blue whales to their ancestral South Georgian foraging grounds, suggesting a rediscovery64. A combination of increasing population numbers and a rediscovery of important habitat, including transmission of this knowledge among the growing population, likely is the cause for large numbers of fin whales using their ancestral feeding grounds again. The fact that it has taken decades since the end of whaling until feeding aggregations of fin whales were observed again for the first time is indicative of the level of depletion and its spatial extent, leaving behind too few mature individuals for a swift recovery and re-occupancy of the habitat64. On a finer scale, some differences in the distribution of fin whales on the feeding grounds is apparent when comparing observed densities to historical catch records. The hotspot area around Elephant Island identified in our study (Fig. 7) matches the area from which feeding aggregations and observations of large numbers of fin whales have repeatedly been reported13,14,16,65, indicating some level of site-fidelity in recent times. However, this area is not reflected by particularly high catch records from the whaling period. This could indicate that fin whales did not concentrate in that particular area during whaling times. However, the discrepancy could also arise from the allocation of catch effort. Elephant Island is located at the outer limits of the former whaling ground and was visited much less by the whalers than the area around the South Shetland Islands, where whaling stations and ports were based. Distant areas of the whaling grounds were only visited on '[…] exceptional occasions, when the whales were unusually scarce around the South Shetland Islands'7. Consequently, low catch records around Elephant Island do not mean that historically fin whales must have been less abundant there.
On the other hand, catch records report highest fin whale catch-numbers around the South Shetland Islands and in the inshore areas of the Bransfield Strait (Fig. 8)7, indicating that fin whale numbers in these areas must have been high. While fin whales seem to have returned to the South Shetland Islands in large numbers, abundance in the Bransfield Strait remains low (this study15,66,67). Today, the inshore waters of the Antarctic Peninsula, including the Bransfield Strait, are dominated by humpback whales (Megaptera novaeangliae)15,67,68,69,70. Humpback whales have recovered from depletion by whaling at a much faster rate than other baleen whale populations in the Southern Hemisphere71. It is possible, that humpback whale dominance has caused fin whales to not reclaim their former feeding grounds in inshore waters, but predominantly using the outer shelf area. This horizontal niche-partitioning of the two species has been suggested previously by Herr et al.15. Another explanation may be that fin whale numbers are still small in comparison to historical times and expansion into ancestral feeding grounds is not yet complete.
Population recovery
The results of our survey represent a snapshot of the minimum number (i.e., uncorrected for availability bias) of fin whales present in the area at the time of the survey. Comparing abundances between survey areas of different size and particularly with arbitrary choices of survey boundaries with regard to animal distribution and population boundaries, is mostly unfavourable. Therefore, density is the key metric for comparisons in time and space, also for comparison of our results with future estimates. However, the abundance estimate of 7909 (95% CI 1047–15,743) individuals suggests a considerable number of fin whales gathering again today in a comparatively small area off the Antarctic Peninsula during austral summer feeding season. In 2000, only 4672 (CV 42.37) whales were estimated fora much larger area comprising the Antarctic Peninsula and Scotia Arc region9. To put these numbers into a broader context, information on population structure, location of breeding grounds and migratory movements are needed. It remains unknown where these animals migrate from and if they belong to one or more breeding populations. Fin whales occur in both, the South Pacific and the South Atlantic72, therefore both Oceans are candidate areas of origin for these fin whales. Mixing of breeding stocks at feeding grounds is known from humpback whales73,74. But the population structure of Southern Hemisphere fin whales is not yet understood75,76,77, and the locations of lower latitude breeding or wintering grounds are unknown78. In the absence of this knowledge, we cannot draw definite conclusions on population recovery. However, if we rule out large-scale shifts of prey as another possible explanation for changes in observed animal abundance, a rediscovery of important habitat by a recovering population remains the most likely explanation for a re-occurrence of high numbers of fin whales at their historic feeding grounds. The Antarctic Peninsula region is known as a highly productive marine area with abundant krill, which has been sustaining large populations of krill predators throughout time79,80,81. Despite a suggested long-term decline in the krill stock82 and interannual fluctuations of abundance83, krill has not seen large scale distributional changes over past decades84 and sufficient biomass would have been available at the feeding grounds any time81. Therefore, changes in distribution and abundance of krill or other prey are unlikely explanations for the re-appearance of large numbers of fin whales at their historical feeding grounds.
Environmental implications
As top predators, whales are indicators for ecosystem health. They are important ecosystem engineers, contributing to the stability and resilience of the ecosystem85. Recent estimates of prey consumption suggest that pre-exploitation populations of baleen whales in the Southern Ocean must have consumed 430 million tonnes of krill annually86, i.e., twice the estimated total biomass of E. superba today87. These new estimates lend additional support to the concept that whales fertilised their own feeding grounds in the Southern Ocean by feeding on iron-rich krill and discharging iron-rich faecal plumes in the surface layer88,89,90,91. In a region where primary productivity is largely limited by iron availability, whales would thus have substantially enhanced phytoplankton growth, boosting food availability for krill91,92. Krill biomass would thus be highly linked to whale abundance, explaining why the predicted 'krill surplus'93 as an effect of the removal of Southern Ocean whales from the ecosystem never materialised89, but krill instead declined82. The recovery of baleen whales and their nutrient recycling services, known as "the whale-pump"91, could thus augment primary productivity and restore ecosystem functions lost during twentieth century whaling85,86. A recovering fin whale population may lead to an increase of Southern Ocean productivity through enhancing iron levels in the surface layer88. By stimulating primary production, whales act as a carbon sink in the Southern Ocean85,94. This is of particular relevance, since the Southern Ocean is a major component of the coupled ocean–atmosphere climate system95, crucial for atmospheric carbon regulation and the most important ocean region for the uptake of anthropogenic CO296,97.
Conclusion
High densities, re-establishment of historical behaviours and the return to ancestral feeding grounds are promising signs for a recovering population. The aggregations documented in this study resemble descriptions of observers from the pre-whaling period: 'Whales' backs and blasts were seen at close intervals quite near to the ship and from horizon to horizon […]'44, raising hope that fin whales are on their way to pre-exploitation numbers. In times of climate change, biodiversity loss and species extinction, the recovery of a large whale population is not only a glimpse of hope; it is also likely to have a stimulating effect on primary production in the Southern Ocean, enhancing CO2 uptake and carbon sink capacities.
Data availability
Scripts and data used in the analyses can be accessed at https://github.com/sviquerat/FinWhaleReturn.
References
Scott Baker, C. & Clapham, P. J. Modelling the past and future of whales and whaling. Trends Ecol. Evol. 19, 365–371. https://doi.org/10.1016/j.tree.2004.05.005 (2004).
Clapham, P. J. & Baker, C. S. in Encyclopedia of Marine Mammals (eds W. F. Perrin, B. Würsig, & J. G. M. Thewissen) Ch. Modern Whaling, 1328–1332 (Academic Press, 2002).
International Whaling Commission. Twenty-Eighth Report of the International Whaling Commission. (International Whaling Commission, Cambridge, 1978).
International Whaling Commission. Thirty-Sixth Report of the International Whaling Commission. (International Whaling Commission, Cambridge, 1986).
Leaper, R. & Miller, C. Management of Antarctic baleen whales amid past exploitation, current threats and complex marine ecosystems. Antarct. Sci. 23, 503–529. https://doi.org/10.1017/s0954102011000708 (2011).
Tulloch, V. J. D., Plagányi, É. E., Matear, R., Brown, C. J. & Richardson, A. J. Ecosystem modelling to quantify the impact of historical whaling on Southern Hemisphere baleen whales. Fish Fish. 19, 117–137. https://doi.org/10.1111/faf.12241 (2018).
Kemp, S. & Bennett, A. G. On the distribution and movements of whales on the South Georgia and South Shetland whaling grounds. Discov. Rep. 6, 165–190 (1932).
Branch, T. A. & Butterworth, D. S. Estimates of abundance south of 60°S for cetacean species sighted frequently on the 1978/79 to 1997/98 IWC/IDCR-SOWER sighting surveys. J. Cetac. Res. Manag. 3, 251–270 (2001).
Reilly, S. et al. Biomass and energy transfer to baleen whales in the South Atlantic sector of the Southern Ocean. Deep Sea Res. Part II 51, 1397–1409. https://doi.org/10.1016/s0967-0645(04)00087-6 (2004).
Cooke, J. G. Balaenoptera physalus. The IUCN Red List of Threatened Species 2018, e.T2478A50349982. https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T2478A50349982.en (2018).
Santora, J. A., Schroeder, I. D. & Loeb, V. J. Spatial assessment of fin whale hotspots and their association with krill within an important Antarctic feeding and fishing ground. Mar. Biol. 161, 2293–2305. https://doi.org/10.1007/s00227-014-2506-7 (2014).
Santora, J. A., Reiss, C. S., Loeb, V. J. & Veit, R. R. Spatial association between hotspots of baleen whales and demographic patterns of Antarctic krill Euphausia superba suggests size-dependent predation. Mar. Ecol. Prog. Ser. 405, 255–269. https://doi.org/10.3354/meps08513 (2010).
Burkhardt, E. et al. Seasonal and diel cycles of fin whale acoustic occurrence near Elephant Island, Antarctica. R Soc. Open Sci. 8, 201142. https://doi.org/10.1098/rsos.201142 (2021).
Joiris, C. R. & Dochy, O. A major autumn feeding ground for fin whales, southern fulmars and grey-headed albatrosses around the South Shetland Islands, Antarctica. Polar Biol. 36, 1649–1658. https://doi.org/10.1007/s00300-013-1383-8 (2013).
Herr, H. et al. Horizontal niche partitioning of humpback and fin whales around the West Antarctic Peninsula: Evidence from a concurrent whale and krill survey. Polar Biol. 39, 799–818. https://doi.org/10.1007/s00300-016-1927-9 (2016).
Viquerat, S. & Herr, H. Mid-summer abundance estimates of fin whales Balaenoptera physalus around the South Orkney Islands and Elephant Island. Endanger. Spec. Res. 32, 515–524. https://doi.org/10.3354/esr00832 (2017).
Meyer, B. & Wessels, W. The Expedition PS112 of the Research Vessel POLARSTERN to the Antarctic Peninsula Region in 2018. 125 p. (Alfred Wegener Institute for Polar and Marine Research, Bremerhaven, 2018). https://doi.org/10.2312/BzPM_0722_2018
Knust, R. Polar research and supply vessel POLARSTERN operated by the Alfred-Wegener-Institute. J. Large-Scale Res. Facil. JLSRF 3, A119. https://doi.org/10.17815/jlsrf-3-163 (2017).
Buckland, S. T. et al. Introduction to Distance Sampling: Estimating Abundance of Biological Populations (Oxford University Press, 2001).
Herr, H. et al. Aerial surveys for Antarctic minke whales (Balaenoptera bonaerensis) reveal sea ice dependent distribution patterns. Ecol Evol 9, 5664–5682. https://doi.org/10.1002/ece3.5149 (2019).
Dorschel, B. et al. Environmental information for a marine ecosystem research approach for the northern Antarctic Peninsula (RV Polarstern expedition PS81, ANT-XXIX/3). Polar Biol. 39, 765–787. https://doi.org/10.1007/s00300-015-1861-2 (2015).
Miller, D. L., Burt, M. L., Rexstad, E. A., Thomas, L. & Gimenez, O. Spatial models for distance sampling data: recent developments and future directions. Methods Ecol. Evol. 4, 1001–1010. https://doi.org/10.1111/2041-210x.12105 (2013).
Hedley, S. L. & Buckland, S. T. Spatial models for line transect sampling. J. Agric. Biol. Environ. Stat. 9, 181–199. https://doi.org/10.1198/1085711043578 (2004).
Hammond, P. S. et al. Estimating the abundance of marine mammal populations. Front. Mar. Sci. https://doi.org/10.3389/fmars.2021.735770 (2021).
Buckland, S. T. et al. Advanced Distance Sampling (Oxford University Press, 2007).
Miller, D. L., Rexstad, E., Thomas, L., Marshall, L. & Laake, J. L. Distance sampling in R. J. Stat. Softw. 89, 1–28. https://doi.org/10.18637/jss.v089.i01 (2019).
Marques, F. F. C. & Buckland, S. T. in Advanced Distance Sampling (eds S. T. Buckland et al.) 31–47 (Oxford University Press, 2004).
Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 19, 716–723 (1974).
Wood, S. N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semparametric generalized linear models. J. R. Stat. Soc. (B) 73, 3–36. https://doi.org/10.1111/j.1467-9868.2010.00749.x (2011).
Wood, S. N., Pya, N. & Saefken, B. Smoothing parameter and model selection for general smooth models (with discussion). J. Am. Stat. Assoc. 111, 1548–1575. https://doi.org/10.1080/01621459.2016.1180986 (2016).
Dorschel, B. et al. The International Bathymetric Chart of the Southern Ocean Version 2 (IBCSO v2). Scientific Data. https://doi.org/10.1038/s41597-022-01366-7 (2022).
Wilson, M. F. J., O’Connell, B., Brown, C., Guinan, J. C. & Grehan, A. J. Multiscale terrain analysis of multibeam bathymetry data for habitat mapping on the continental slope. Mar. Geod. 30, 3–35. https://doi.org/10.1080/01490410701295962 (2007).
raster: Geographic Data Analysis and Modelling. R package version 3.4–5 (2020).
Shelton, A. O., Thorson, J. T., Ward, E. J., Feist, B. E. & Cooper, A. Spatial semiparametric models improve estimates of species abundance and distribution. Can. J. Fish. Aquat. Sci. 71, 1655–1666. https://doi.org/10.1139/cjfas-2013-0508 (2014).
Dunn, P. K. & Smyth, G. K. Series evaluation of Tweedie exponential dispersion model densities. Statiustics Comput. 15, 267–280 (2005).
Gu, C. & Wahba, G. Minimizing GCV/GML scores with multiple smoothing parameters via the newton method. SIAM J. Sci. Stat. Comput. 12, 383–398. https://doi.org/10.1137/0912021 (1991).
Hobson, E. S. Feeding behaviour in three species of sharks. Pac. Sci. 17, 171–194 (1963).
Weeks, S., Magno-Canto, M., Jaine, F., Brodie, J. & Richardson, A. Unique sequence of events triggers manta ray feeding frenzy in the Southern Great Barrier Reef, Australia. Remote Sens. 7, 3138–3152. https://doi.org/10.3390/rs70303138 (2015).
Montero-Quintana, A. N., Ocampo-Valdez, C. F., Vázquez-Haikin, J. A., Sosa-Nishizaki, O. & Osorio-Beristain, M. Whale shark (Rhincodon typus) predatory flexible feeding behaviors on schooling fish. J. Ethol. 39, 399–410. https://doi.org/10.1007/s10164-021-00717-y (2021).
Findlay, K. P. et al. Humpback whale “super-groups”—A novel low-latitude feeding behaviour of Southern Hemisphere humpback whales (Megaptera novaeangliae) in the Benguela Upwelling System. PLoS ONE 12, e0172002. https://doi.org/10.1371/journal.pone.0172002 (2017).
Pirotta, V., Owen, K., Donnelly, D., Brasier, M. J. & Harcourt, R. First evidence of bubble-net feeding and the formation of ‘super-groups’ by the east Australian population of humpback whales during their southward migration. Aquat. Conserv. Mar. Freshwat. Ecosyst. 31, 2412–2419. https://doi.org/10.1002/aqc.3621 (2021).
Baines, M., Reichelt, M. & Griffin, D. An autumn aggregation of fin (Balaenoptera physalus) and blue whales (B. musculus) in the Porcupine Seabight, southwest of Ireland. Deep Sea Res. Part II Top. Stud. Oceanogr. 141, 168–177, https://doi.org/10.1016/j.dsr2.2017.03.007 (2017).
Ladrón de Guevara P., P., Lavaniegos, B. E. & Heckel, G. Fin whales (Balaenoptera physalus) foraging on daytime surface swarms of the euphausiid Nyctiphanes simplex in Ballenas Channel, Gulf of California, Mexico. J. Mammal. 89, 559–566 (2008).
Bruce, W. S. Some observations on Antarctic cetacea. In: Report on the Scientific results of the voyage of S.Y. ‘Scotia’ during the years 1902, 1903, and 1904, under the leadership of William S. Bruce, 491–505 (1915).
Mackintosh, N. A. & Wheeler, J. F. G. Southern blue and fin whales. Discov. Rep. I, 257–540 (1929).
Jackson, J. A. et al. Have whales returned to a historical hotspot of industrial whaling? The pattern of southern right whale Eubalaena australis recovery at South Georgia. Endang. Spec. Res. 43, 323–339. https://doi.org/10.3354/esr01072 (2020).
Goldbogen, J. A., Pyenson, N. D. & Shadwick, R. E. Big gulps require high drag for fin whale lunge feeding. Mar. Ecol. Prog. Ser. 349, 289–301. https://doi.org/10.3354/meps07066 (2007).
Goldbogen, J. A. et al. Scaling of lunge-feeding performance in rorqual whales: mass-specific energy expenditure increases with body size and progressively limits diving capacity. Funct. Ecol. 26, 216–226. https://doi.org/10.1111/j.1365-2435.2011.01905.x (2012).
Acevedo-Gutierrez, A., Croll, D. A. & Tershy, B. R. High feeding costs limit dive time in the largest whales. J Exp Biol 205, 1747–1753. https://doi.org/10.1242/jeb.205.12.1747 (2002).
Keen, E. M. Aggregative and feeding thresholds of sympatric rorqual whales within a fjord system. Ecosphere 8, e01702. https://doi.org/10.1002/ecs2.1702 (2017).
Veit, R. R. & Harrison, N. M. Positive interactions among foraging seabirds, marine mammals and fishes and implications for their conservation. Front. Ecol. Evol. https://doi.org/10.3389/fevo.2017.00121 (2017).
Laran, S. et al. A comprehensive survey of pelagic megafauna: their distribution, densities, and taxonomic richness in the tropical Southwest Indian Ocean. Front. Mar. Sci. https://doi.org/10.3389/fmars.2017.00139 (2017).
Campbell, G. S. et al. Inter-annual and seasonal trends in cetacean distribution, density and abundance off southern California. Deep Sea Res. Part II Top. Stud. Oceanogr. 112, 143–157. https://doi.org/10.1016/j.dsr2.2014.10.008 (2015).
Heide-Jørgensen, M. P. et al. Estimates of large whale abundance in West Greenland waters from an aerial survey in 2005. J. Cetac. Res. Manag. 10, 119–129 (2008).
Panigada, S. et al. Estimating cetacean density and abundance in the Central and Western Mediterranean Sea through aerial surveys: Implications for management. Deep Sea Res. Part II Top. Stud. Oceanogr. 141, 41–58. https://doi.org/10.1016/j.dsr2.2017.04.018 (2017).
Forcada, J., Aguilar, A., Hammond, P., Pastor, X. & Aguilar, R. Distribution and abundance of fin whales (Balaenoptera physalus) in the western Mediterranean sea during the summer. J. Zool. 238, 23–34. https://doi.org/10.1111/j.1469-7998.1996.tb05377.x (2009).
Clapham, P. J., Aguilar, A. & Hatch, L. T. Determining spatial and temporal scales for management: lessons from whaling. Mar. Mamm. Sci. 24, 183–201. https://doi.org/10.1111/j.1748-7692.2007.00175.x (2008).
Baker, C. S. et al. Strong maternal fidelity and natal philopatry shape genetic structure in North Pacific humpback whales. Mar. Ecol. Prog. Ser. 494, 291–306. https://doi.org/10.3354/meps10508 (2013).
Carroll, E. L. et al. Cultural traditions across a migratory network shape the genetic structure of southern right whales around Australia and New Zealand. Sci. Rep. 5, 16182. https://doi.org/10.1038/srep16182 (2015).
Valenzuela, L. O., Sironi, M., Rowntree, V. J. & Seger, J. Isotopic and genetic evidence for culturally inherited site fidelity to feeding grounds in southern right whales (Eubalaena australis). Mol. Ecol. 18, 782–791. https://doi.org/10.1111/j.1365-294X.2008.04069.x (2009).
Barendse, J., Best, P. B., Carvalho, I. & Pomilla, C. Mother knows best: occurrence and associations of resighted humpback whales suggest maternally derived fidelity to a Southern Hemisphere coastal feeding ground. PLoS ONE 8, e81238. https://doi.org/10.1371/journal.pone.0081238 (2013).
Richard, G. et al. Cultural transmission of fine-scale fidelity to feeding sites may shape humpback whale genetic diversity in Russian Pacific waters. J. Hered. 109, 724–734. https://doi.org/10.1093/jhered/esy033 (2018).
Carroll, E. L. et al. Reestablishment of former wintering grounds by New Zealand southern right whales. Mar. Mamm. Sci. 30, 206–220. https://doi.org/10.1111/mms.12031 (2014).
Calderan, S. V. et al. South Georgia blue whales five decades after the end of whaling. Endang. Spec. Res. 43, 359–373. https://doi.org/10.3354/esr01077 (2020).
Santora, J. A. & Veit, R. R. Spatio-temporal persistence of top predator hotspots near the Antarctic Peninsula. Mar. Ecol. Prog. Ser. 487, 287–304. https://doi.org/10.3354/meps10350 (2013).
Scheidat, M. et al. Cetacean surveys in the Southern Ocean using icebreaker-supported helicopters. Polar Biol. 34, 1513–1522. https://doi.org/10.1007/s00300-011-1010-5 (2011).
Williams, R., Hedley, S. L. & Hammond, P. S. Modeling distribution and abundance of Antarctic baleen whales using ships of opportunity. Ecol. Soc. 11, [online] http://www.ecologyandsociety.org/vol11/iss11/art11/ (2006).
Secchi, E. et al. Encounter rates of whales around the Antarctic peninsula with special reference to humpback whales, Megaptera novaeangliae, in the Gerlache Strait 1997–98 to 1999–2000. Mem. Qld. Mus. 47, 571–578 (2001).
Thiele, D. et al. Seasonal variability in whale encounters in the Western Antarctic Peninsula. Deep Sea Res. Part II Top. Stud. Oceanogr. 51, 2311–2325. https://doi.org/10.1016/j.dsr2.2004.07.007 (2004).
Johnston, D. W., Friedlaender, A. S., Read, A. J. & Nowacek, D. P. Initial density estimates of humpback whales Megaptera novaeangliae in the inshore waters of the western Antarctic Peninsula during the late autumn. Endang. Spec. Res. 18, 63–71. https://doi.org/10.3354/esr00395 (2012).
Zerbini, A. N. et al. Assessing the recovery of an Antarctic predator from historical exploitation. R Soc Open Sci 6, 190368. https://doi.org/10.1098/rsos.190368 (2019).
Mackintosh, N. A. The distribution of southern blue and fin whales, in Whales, dolphins, and porpoises. (ed K. S. Norris) Ch. 8, 125–144 (University of California Press, 1966).
Schmitt, N. et al. Mixed-stock analysis of humpback whales (Megaptera novaeangliae) on Antarctic feeding grounds. J. Cetac. Res. Manag. 14, 141–157 (2014).
Schall, E. et al. Humpback whale song recordings suggest common feeding ground occupation by multiple populations. Sci. Rep. 11, 18806. https://doi.org/10.1038/s41598-021-98295-z (2021).
Archer, F. I. et al. Revision of fin whale Balaenoptera physalus (Linnaeus, 1758) subspecies using genetics. J. Mammal. 100, 1653–1670. https://doi.org/10.1093/jmammal/gyz121 (2019).
Archer, F. I. et al. Mitogenomic phylogenetics of fin whales (Balaenoptera physalus spp.): Genetic evidence for revision of subspecies. PLoS ONE 8, e63396. https://doi.org/10.1371/journal.pone.0063396 (2013).
Cabrera, A. A. et al. Fin whale (Balaenoptera physalus) mitogenomics: A cautionary tale of defining sub-species from mitochondrial sequence monophyly. Mol. Phylogenet. Evol. 135, 86–97. https://doi.org/10.1016/j.ympev.2019.02.003 (2019).
Edwards, E. F., Hall, C., Moore, T. J., Sheredy, C. & Redfern, J. V. Global distribution of fin whales Balaenoptera physalus in the post-whaling era (1980–2012). Mammal. Rev. 45, 197–214. https://doi.org/10.1111/mam.12048 (2015).
Knox, G. A. The key role of krill in the ecosystem of the Southern Ocean with special reference to the Convention on the Conservation of Antarctic Marine Living Resources. Ocean Manag. 9, 113–156 (1984).
Ducklow, H. W. et al. Marine pelagic ecosystems: the west Antarctic Peninsula. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362, 67–94. https://doi.org/10.1098/rstb.2006.1955 (2007).
Atkinson, A. et al. Krill (Euphausia superba) distribution contracts southward during rapid regional warming. Nat. Clim. Change 9, 142–147. https://doi.org/10.1038/s41558-018-0370-z (2019).
Benton, M. J., Tverdokhlebov, V. P. & Surkov, M. V. Ecosystem remodelling among vertebrates at the Permian-Triassic boundary in Russia. Nature 432, 97–100. https://doi.org/10.1038/nature02950 (2004).
Siegel, V., Reiss, C. S., Dietrich, K. S., Haraldsson, M. & Rohardt, G. Distribution and abundance of Antarctic krill (Euphausia superba) along the Antarctic Peninsula. Deep Sea Res. Part I Oceanogr. Res. Pap. 77, 63–74. https://doi.org/10.1016/j.dsr.2013.02.005 (2013).
Siegel, V. & Watkins, L. Distribution, Biomass and Demography of Antarctic Krill, Euphausia superba, in Biology and Ecology of Antarctic Krill Advances in Polar Ecology (ed Volker Siegel) Ch. 2, 21–101 (Springer, 2016).
Roman, J. et al. Whales as marine ecosystem engineers. Front. Ecol. Environ. 12, 377–385. https://doi.org/10.1890/130220 (2014).
Savoca, M. S. et al. Baleen whale prey consumption based on high-resolution foraging measurements. Nature 599, 85–90. https://doi.org/10.1038/s41586-021-03991-5 (2021).
Atkinson, A., Siegel, V., Pakhomov, E. A., Jessopp, M. J. & Loeb, V. A re-appraisal of the total biomass and annual production of Antarctic krill. Deep Sea Res. Part I Oceangr. Res. Pap. 56, 727–740. https://doi.org/10.1016/j.dsr.2008.12.007 (2009).
Nicol, S. et al. Southern Ocean iron fertilization by baleen whales and Antarctic krill. Fish Fish. 11, 203–209. https://doi.org/10.1111/j.1467-2979.2010.00356.x (2010).
Lavery, T. J. et al. Whales sustain fisheries: Blue whales stimulate primary production in the Southern Ocean. Mar. Mamm. Sci. 30, 888–904. https://doi.org/10.1111/mms.12108 (2014).
Smetacek, V. Are declining Antarctic krill stocks a result of global warming or of the decimation of the whales? in Effects of global warming on polar ecosystems (ed Carlos M. Duarte) (Fundación BBVA, 2008).
Roman, J. & McCarthy, J. J. The whale pump: marine mammals enhance primary productivity in a coastal basin. PLoS ONE 5, e13255. https://doi.org/10.1371/journal.pone.0013255 (2010).
Smetacek, V. A whale of an appetite revealed by analysis of prey consumption. Nature 599, 33–34. https://doi.org/10.1038/d41586-021-02951-3 (2021).
Laws, R. M. Seals and whales of the Southern Ocean. Philios. Trans. R. Soc. Lond. B 279, 81–96 (1977).
Lavery, T. J. et al. Iron defecation by sperm whales stimulates carbon export in the Southern Ocean. Proc. Biol. Sci. 277, 3527–3531. https://doi.org/10.1098/rspb.2010.0863 (2010).
Cunningham, S. A. Transport and variability of the Antarctic Circumpolar Current in Drake Passage. J. Geophys. Res. https://doi.org/10.1029/2001jc001147 (2003).
Frölicher, T. L. et al. Dominance of the Southern Ocean in Anthropogenic Carbon and Heat Uptake in CMIP5 Models. J. Clim. 28, 862–886. https://doi.org/10.1175/jcli-d-14-00117.1 (2015).
Landschutzer, P. et al. The reinvigoration of the Southern Ocean carbon sink. Science 349, 1221–1224. https://doi.org/10.1126/science.aab2620 (2015).
Acknowledgements
We thank the crews of RV Polarstern, Pelagic Australis and the helicopter team of expedition PS112 for facilitating our data collection. We are grateful for the help of Boris Dorschel, Cleone Fox, Rob Hawthorne and James Loudon and thank the BBC Seven Worlds, One Planet team. This work was supported by the Antarctic Wildlife Research (AWR) Fund, by the Southern Ocean Research Partnership (IWC-SORP) and by the German Research Foundation (DFG) within the framework of the priority programme "Antarctic Research with comparative investigations in Arctic ice areas" SPP 1158 by Grant HE 5696/3-1.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
H.H. and S.V. conceived the idea for the study. H.H., S.V., F.D. and B.M. designed, planned and realized the fieldwork. H.H., S.V., A.L., B.G., L.W., T.G., D.B. collected and processed the data. H.H. and S.V. analysed the data. S.V. performed the statistical computations. H.H. wrote the draft manuscript and produced the maps. All authors provided critical feedback and approved of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video S1.
Supplementary Video S2.
Supplementary Video S3.
Supplementary Video S4.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Herr, H., Viquerat, S., Devas, F. et al. Return of large fin whale feeding aggregations to historical whaling grounds in the Southern Ocean. Sci Rep 12, 9458 (2022). https://doi.org/10.1038/s41598-022-13798-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-13798-7